Pitch-Patch
Rotator Cuff Tissue Augmentation
Rotator Cuff Repair – Confident Solutions
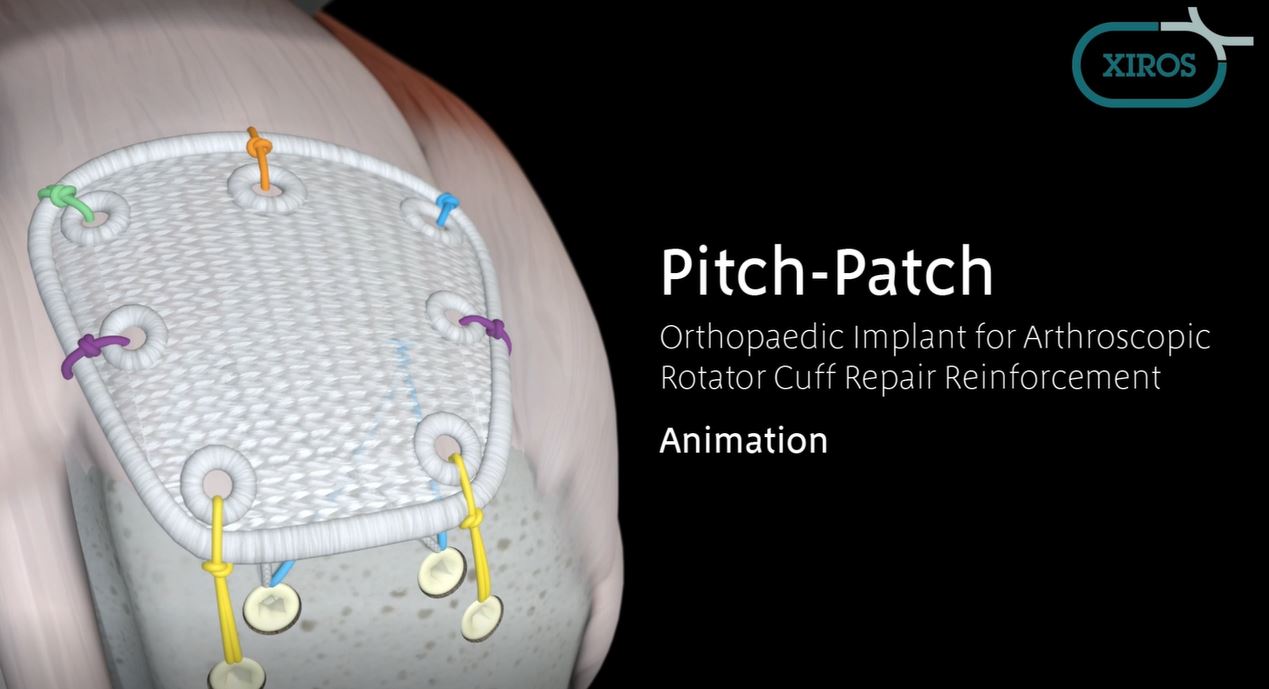
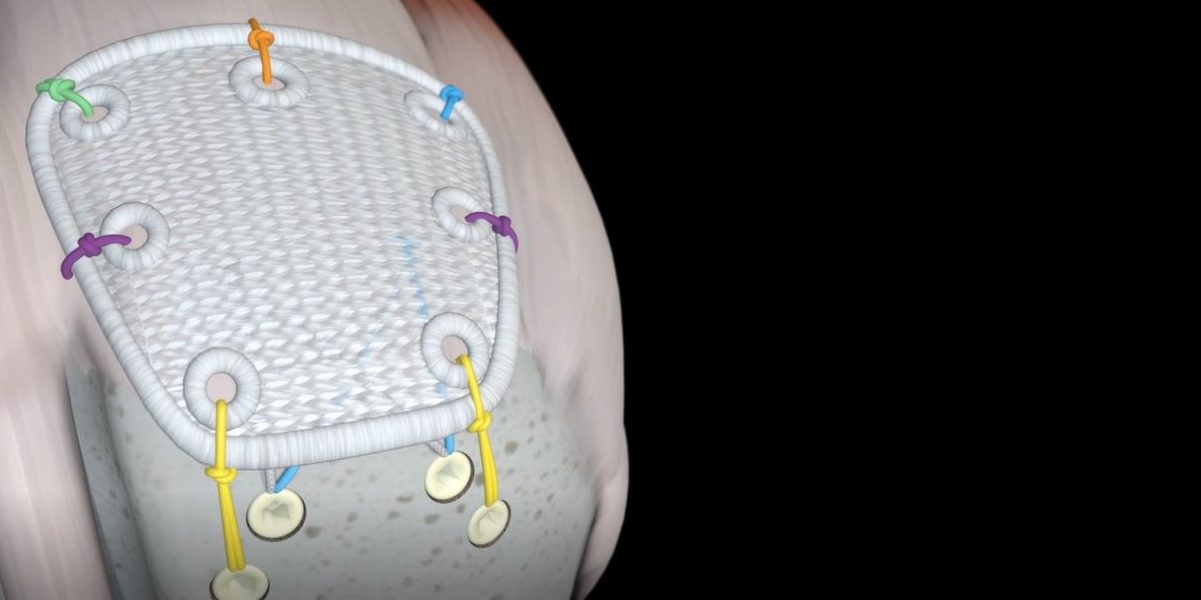
Pitch-Patch Rotator Cuff tissue augmentation device is a non-absorbable, sterile, polyester, open-weave textile with reinforced borders and suture holes.
-

High Strength
-

Long History of Medical Use
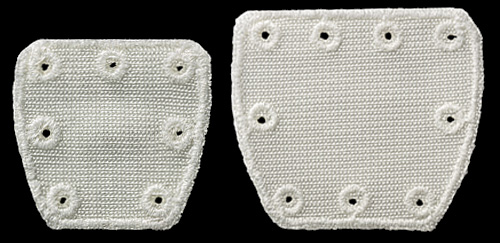
Pitch-Patch is a polyester patch for reinforcement of the rotator cuff following repair by sutures or suture anchors. The shape is designed to fit the anatomy. It features a reinforced border and incorporates reinforced, prepared holes for the sutures, up to size #5. It is available in two sizes to cover different tear sizes of the rotator cuff: 30 mm x 20 mm and 35 mm x 25 mm.
Polyester implants have a long history of routine use in joint surgery. They are well tolerated and show little foreign body tissue reaction. Unlike biological tissue, the polyester material used for Pitch-Patch does not cause any immunological reaction. Testing of this special design has shown a very high average strength of over 355 N for the standard patch.
Pitch-Patch can be applied using an open or arthroscopic technique for the reinforcement of the rotator cuff. Pitch-Patch is not intended for stand-alone bridging (replacement of the cuff with insufficient closure).
Indications
- Pitch-Patch is a single-use device intended to be used for reinforcement of the rotator cuff following or during repair by suture or suture anchors, where weakness exists in the soft tissue.
- Pitch-Patch is not intended to replace normal body structure or provide the full mechanical strength to support the rotator cuff. Sutures, used to repair the tear, and sutures or bone anchors, used to attach the tissue to the bone, provide mechanical strength for the repair.
- Pitch-Patch reinforces the rotator cuff and provides a scaffold that is incorporated into the patient’s own tissue.
- Pitch-Patch is indicated for patients requiring reinforcement of the rotator cuff where either the tear cannot be completely repaired using normal methods and/or the quality of the soft tissue is poor. Further information regarding indications and contraindications are available in the Instructions-for-Use (IFU) LAB 300.
How does it work
- The surgeon repairs the rotator cuff with his/her preferred technique.
- Pitch Patch is sutured to the repaired rotator cuff tendon using a combination of free sutures and soft tissue anchors.
- The Pitch Patch device augments the tissues natural strength and function through the healing process.
- See the Pitch Patch Instructions for Use (IFU) and Surgical Technique Manual (STM) for additional information and instruction.
Although most patients benefit from current techniques of RC repair, retears remain the biggest problem after surgery. Hence, considering the high costs associated with surgical intervention followed by lengthy rehabilitation, there is a constant need to devise better ways to avoid construct failure and consecutive revision surgery.2,3
In radiologic follow-up, the Leuzinger, et al, study showed that the retear rate of 14% compares favorably with the existing literature in standard nonaugmented RC repairs.1
Order Codes
- 102 -1090 Pitch-Patch Tissue Reinforcement, Standard (30 mm x 20 mm) (sterile, single-use)
- 102 -1091 Pitch-Patch Tissue Reinforcement, X-Large (35 mm x 25 mm) (sterile, single-use)
FEATURES AND BENEFITS
- High strength construct of polyester to augment Rotator Cuff Repairs
- Surgical techniques for Arthroscopic and Open procedures
- Polyester has a long history of safe and efficacious use in surgical procedures
- Predetermined elongation which is anatomically aligned
- Woven polyester implant utilizes medical manufacturing techniques supported by over 30 years of data
- Open-weave allows tissue ingrowth
- Reinforced eyelets to prevent suture pull-through
- Flexible technique to accommodate anatomic variations
Product Documents
- Application of a new polyester patch in arthroscopic massive rotator cuff repair-a prospective cohort study
- Pitch-Patch Product Sheet
- eIFU (Instructions for Use) Documents
CONTRAINDICATIONS
- Do not use Pitch-Patch as a bridging device.
- Do not use Pitch-Patch if there is any evidence of infection.
- Do not use Pitch-Patch in patients with known hypersensitivity to implant materials. If the patient is suspected of having any foreign body sensitivity, appropriate tests must be made prior to implantation.
- Do not use Pitch-Patch in patients who are skeletally immature. It will not elongate as the patient grows.
Additional Information
Please note: Regulatory approval for products mentioned on this website varies from country to country. For further information, and for details of local distributors and agents, contact our Sales and Marketing team at enquiries@xirosna.com. All data listed on this page is held at Xiros and can be requested by contacting enquiries@xirosna.com.
To request the full range of product documentation or to view any of these documents in a language other than English, please contact us for availability. Always refer to the Instructions for Use for detailed information and essential warnings and precautions.
- 1. Daniel Smolen, MDa,b, Nicolas Haffner, MDb,c, Rainer Mittermayr, MDb,d,*, Florian Hess, MDe, Christoph Sternberg, MDa, Jan Leuzinger, MDa; Application of a new polyester patch in arthroscopic massive rotator cuff repair – a prospective cohort study; J Shoulder Elbow Surg (2020) 29, e11-e2. https://doi.org/10.1016/j.jse.2019.05.015
aEtzel Clinic, Pfaeffikon, Switzerland
bLudwig Boltzmann Institute for Experimental and Clinical Traumatology, Vienna, Austria
cOrthopedic Hospital Gersthof, Vienna, Austria
dTrauma Center of the Workmen Compensation Board, Vienna, Austria
eDepartment of Orthopedic Surgery and Traumatology, Kantonsspital Frauenfeld, Frauenfeld, Switzerland
*Reprint requests
- 2. Iyengar JJ, Samagh SP, Schairer W, Singh G, Valone FH, Feeley BT. Current trends in rotator cuff repair: surgical technique, setting, and cost. Arthroscopy 2014;30:284-8. https://doi.org/10.1016/j.arthro.2013.11.018
- 3. Mather RC, Koenig L, Acevedo D, Dall TM, Gallo P, Romeo A, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am 2013;95:1993-2000. https://doi.org/10.2106/JBJS.L.01495
 Xiros
Xiros